advantages of marmosets as model organisms for neuroscience research
The common marmoset has seen a rapid emergence as an ideal model organism for neuroscience and biomedical science over the last few years due to several physiological and logistical advantages. Their small size and ease of handling offers the convenience of laboratory rodent models such as rats and mice. However, unlike rodents, marmosets exhibit the shared physiological, behavioral and cognitive characteristics that are unique to primates, including the core functional architecture and organization of our nervous system. This unique complement of characteristics affords the exciting opportunity to feasibly utilize a primate species to model many of the diseases that afflict humans, ranging from those that affect humans at specific times in life – including both developmentally and during aging – to neuropsychiatric disorders that impact uniquely primate properties of our brain. Major advantages of marmosets for neuroscience research are outlined below.
small size and easy handling
The first advantage of marmosets over other NHP models such as macaques, is their small size, which is similar to lab rats. Adult marmosets are between 15 and 25cm high, excluding the tail, and weigh between 250 and 550 grams (obese animals may weigh more). These features make marmosets easier to handle than larger NHPs, requiring less cost and skill in their maintenance (eg. caging, feeding). Furthermore, due to their small size, marmosets require limited amounts of test compounds during experiments, a marked advantage when testing precious materials.
Easier handling is also attributed to the fact that to date, no fatal zoonotic diseases that can be transmitted to humans have been identified in marmosets, an advantage over macaques who can harbor herpes B.
High reproductive efficiency
Another advantage of marmosets is their high reproductive efficiency. Marmosets are easily bred in captivity and demonstrate the highest reproductive efficiency of any anthropoid primate.
They reach sexual maturity at 1.5 years old and produce 2-3 offspring every 5-6 months. If an adult female is in breeding for 6 years, she will give birth about 12 times and will produce roughly 24 infants.
| Reproductive trait | Rat | Marmoset | Macaque |
|---|---|---|---|
| Sexual maturity | 10-12 weeks | 1.5 years | 3 years |
| Litter size | 5-10 offspring | 2-3 offspring | 1 offspring |
| Gestation period | 21-23 days | 145 days | 165 days |
| Delivery interval | 2 months | 5 months | 1-2 years |
As an example, 10 macaque breeding females will, in two years’ time, produce a maximum of 20 offspring, all of which will be immature (i.e. the reproductive population will not have increased during that time). In contrast, in the same 2-year period, 10 marmoset breeding females will produce an average of 60 offspring and the reproductive population will have tripled, as marmosets reach reproductive maturity by 14-18 months of age.
Fast maturation and short life span
Marmosets offer unique opportunities to study and understand biomedical processes that have not been feasible to model in other NHPs. The fast maturation and relatively short life span of marmosets makes them a valuable resource to study developmental, chronic and aging related disease, which are among the most pressing US human health concerns. Marmosets reach sexual maturity at around 1.5 years and by 8-13 years of age show signs of age-related pathologies such as an increase in cancers, amyloidosis, pathogenic tau accumulation, diabetes and renal diseases, a decrease in lean muscle mass, hearing loss as well as declines in cognitive and motor function with increased age.
Notably, the time period required to move from early to late life (i.e. to advance through the stages of development, aging or chronic disease) is within the range of a typical, NIH-funded project. Macaques on the other hand do not reach sexual maturity and geriatric age until around 3 and 20 years, respectively.
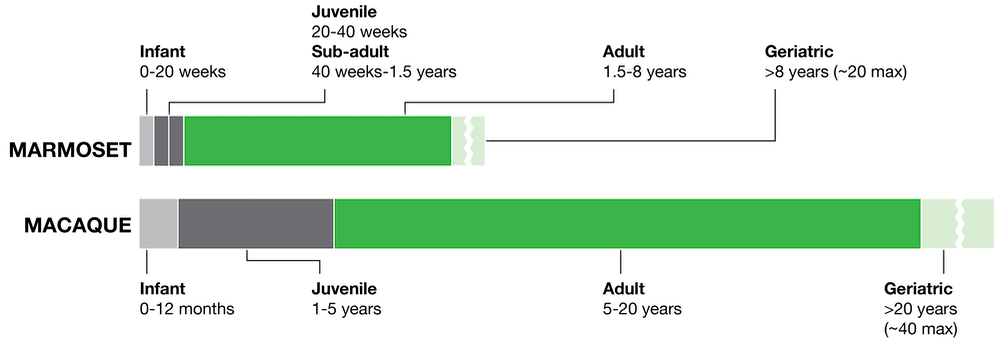
Furthermore, like humans, marmosets are typically group-housed with pair-bonded parents and multiple litters of offspring, making it possible to observe the process of aging across two generations of animals in the same cage. This is a particularly attractive feature for transgenic models in which disease onset may depend on aging factors, such as neurodegenerative disorders like Alzheimer's Disease and Parkinson's Disease.
brain anatomy and function
Although the marmoset brain is small, it retains anatomical and functional organization specific to primates, including humans. This includes a large neocortex, granular prefrontal cortex, expanded visual and auditory cortical fields and reduced olfactory regions. Like humans, the brain of marmosets have a large amount of white matter, a feature that makes them promising as a translational model of brain disease. The marmoset brain is also well suited for studying circuit wiring and connectomics in a complex NHP because it does not pose the same big data challenge for computational tools as the macaque brain which is several magnitudes larger.
In addition, in contrast to the large and convoluted macaque brain, the marmoset cortex is lissencephalic (i.e. smooth) like a rodent's. This greatly facilitates the mapping of functional brain areas by neuroimaging techniques, such as fMRI and optical imaging, as well as by electrophysiology, with high spatial resolution as all of the neocortical fields are accessible directly below the skull rather than within sulci. Although it is important to note that the lissencephalic brain is dissimilar from humans.
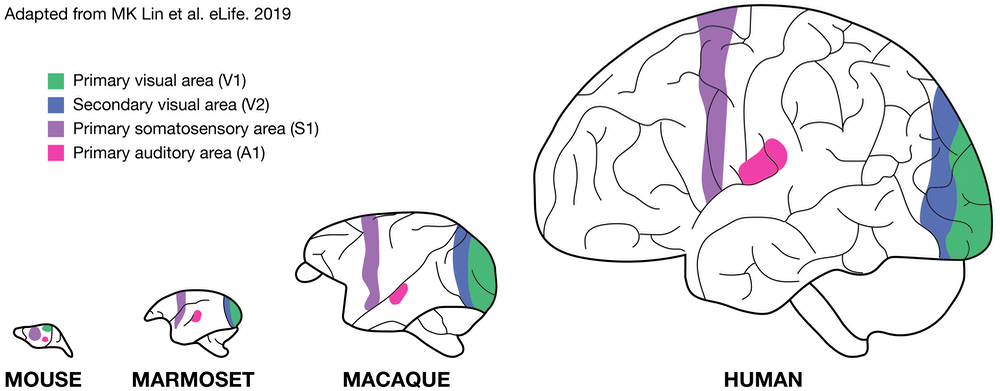
comparable cognitive characteristics to humans
Primates are distinguished from other animals by the breadth and sophistication of our sociality, including the dynamic models we develop for social decision-making to effectively navigate the complexities of these social landscapes. While marmosets share these attributes with other primates, their societies also exhibit characteristics that are typical of humans yet rare in other primates.
Marmosets, for example, pair-bond and cooperatively care for their offspring. Cooperation occurs frequently in marmoset society and the species displays high levels of prosociality. Several experiments show that marmosets also utilize imitation, a distinct social learning mechanism that is rare amongst primates as it had previously only been reported in humans and chimpanzees.
Marmosets are also highly vocal, engaging in near tonic levels of conversational exchanges with conspecifics by utilizing turn-taking mechanisms that are learned during development. While vocal communication is critical to mediating their social interactions, marmosets also utilize diverse visual signals – including facial expressions – as a parallel social signaling system, similarly to humans.
Furthermore, marmosets have the ability to perform cognitive tasks similar to those often used in human studies, such as visual discrimination and reversal learning.